CSIR-CCMB scientists uncover how protein flexibility enables multitasking in plants | Hyderabad News

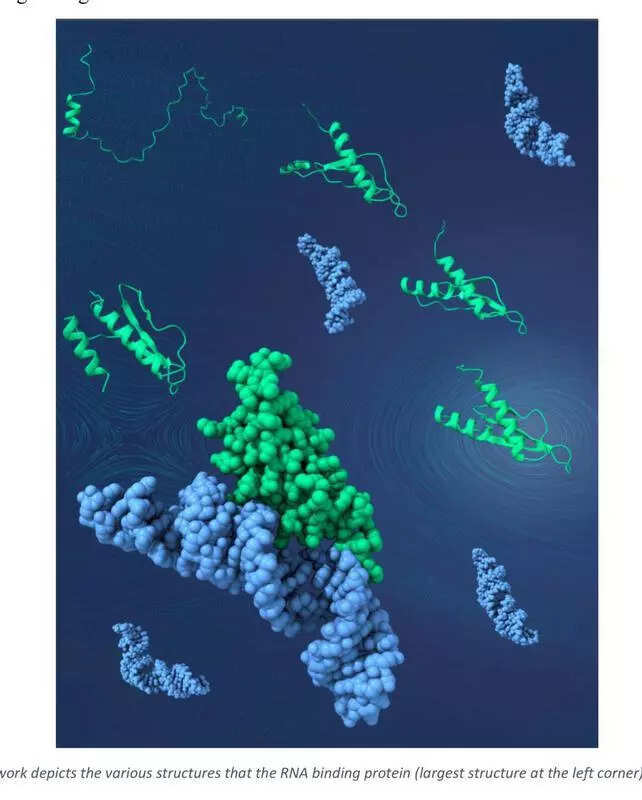
Hyderabad: New research from Council of Scientific & Industrial Research (CSIR) – Centre for Cellular and Molecular Biology (CCMB) has revealed that proteins do not always rely on a fixed three-dimensional shape to perform their functions.Instead, their structures can be flexible, enabling them to carry out multiple roles. This insight reshapes our understanding ofprotein dynamicsin biological systems.The study provides a plausible explanation for how plants fine-tune RNA processing without expanding their protein repertoire, particularly in the absence of adaptive immunity. These findings could pave the way for advances in medicine, agriculture, and biotechnology by assisting scientists in designing proteins capable of multitasking more efficiently.According to a study published in the Journal of the American Chemical Society, two structurally identical plant proteins exhibit varied substrate specificity due to differences in flexibility.One of the proteins, being more flexible, can bind to a broader range of substrate RNA molecules by dynamically rearranging itself to accommodate the shapes of its molecular partners without compromising stability, which is crucial for gene regulation.An artwork illustrates the various structures the RNA-binding protein (shown as the largest structure in the left corner) can interact with. Using a powerful technique called nuclear magnetic resonance (NMR) spectroscopy, combined with sophisticated computational methods, scientists identified tiny populations, just 1%, of protein structures that temporarily switch into different shapes. These rare transitions are essential for recognising diverse RNA forms and help explain how plants manage complex gene regulation using fewer proteins.Dr Mandar V Deshmukh, lead author of the study, said, “What we have shown is that a protein’s ability to change shape, even slightly, can be just as important as its structure.” He added, “By capturing the fleeting, dynamic states of these proteins, we’ve demonstrated that their capacity to transiently rearrange their structure by flexing and adjusting during interaction gives them a functional advantage in complex cellular environments. This enables organisms to regulate genes efficiently under varying conditions. It could transform how we think about developing new medicines or enhancing plant traits.”The study also highlights how subtle changes in a protein’s sequence, within just a few non-active site amino acid residues, can lead to significant functional differences. This underscores the importance of studying both structure and dynamics, particularly for proteins that are potential drug targets.”Our results reveal nature’s originality in designing a unique approach to grant promiscuity to a few proteins,” said Debadutta Patra and Jaydeep Paul, joint first authors of the study.